
Learn more about the providers at Texas Spine and Scoliosis, the only spine specialized neurosurgery, orthopedic surgery, and non-surgical rehabilitation group in the central Texas area.
NOTICE: Our main Austin clinic location has moved to 1004 West 32nd Street. Click here for a map.
Most juvenile and adolescent idiopathic (genetic) scoliosis can be treated with observation and bracing. Patients who are candidates for brace treatment in particular has been the beneficiary of recent Level One (BRAIST) data asserting the effectiveness of bracing in controlling scoliosis up to 50% of the time compared to non braced patients. However, in patients in which bracing is not effective, or who are bracer intolerant.
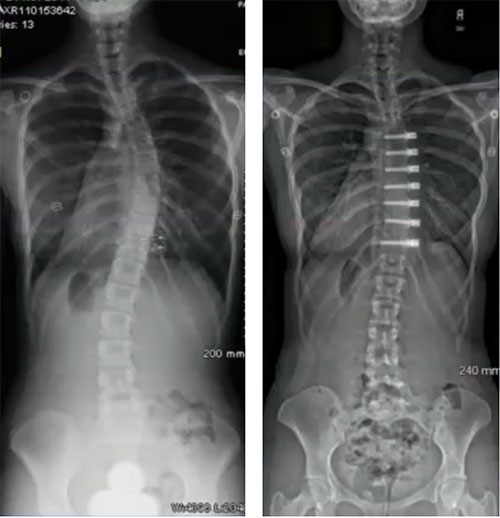
There are new minimally invasive procedures that are fusionless that can be used to treat patients with progressive scoliosis, brace resistant scoliosis, or brace intolerant children. Both access the spine through a tiny incisions (VATS) near the ribs and/or a minimally invasive lateral approach to the spine.
Both techniques work on one side of the curve to prevent it from worsening, and in the case vertebral body tethering actually offer the benefit of correction during the growth spurt. Sometimes it may be the only surgery needed. A second benefit of these fusionless, minimally invasive techniques is that they don’t burn any bridges, and if necessary, more traditional corrective instrumentation can be used later on.
Vertebral Body Tethering is FDA approved as of the Fall of 2019. Dr. Matthew Geck and ScoliosisTexas have HDE/IRB approval and site approval to perform the Zimmer Vertebral Body Tethering System. In addition, we are an approved enrolling site for other tethering systems as well that my suit your child’s situation better.
Vertebral body tethering system is indicated for skeletally immature patients that require surgical treatment to obtain and maintain correction of progressive idiopathic scoliosis, with a major Cobb angle of 35 to 65 degrees whose osseous structure is dimensionally adequate to accommodate screw fixation, as determined by radiographic imaging. Patients should have failed bracing and/or be intolerant to brace wear. The clinical data suggests Vertebral Body Tethering System provides benefit related to prevention of spinal curve progression and avoidance of spinal fusion.
A: This technique places a compressive force over the convex side of the spine (slowing down growth) to permit the concave side of the spine to relatively grow more and create a straighter spine. Prior to the introduction of the vertebral body tether, which uses screws placed into the vertebral body, modulating growth of the concave and convex side of the spine was accomplished with staples. These staples were also placed anteriorly, but instead of being placed in the middle of the vertebral body they were placed across the disc spaces between each vertebral body.
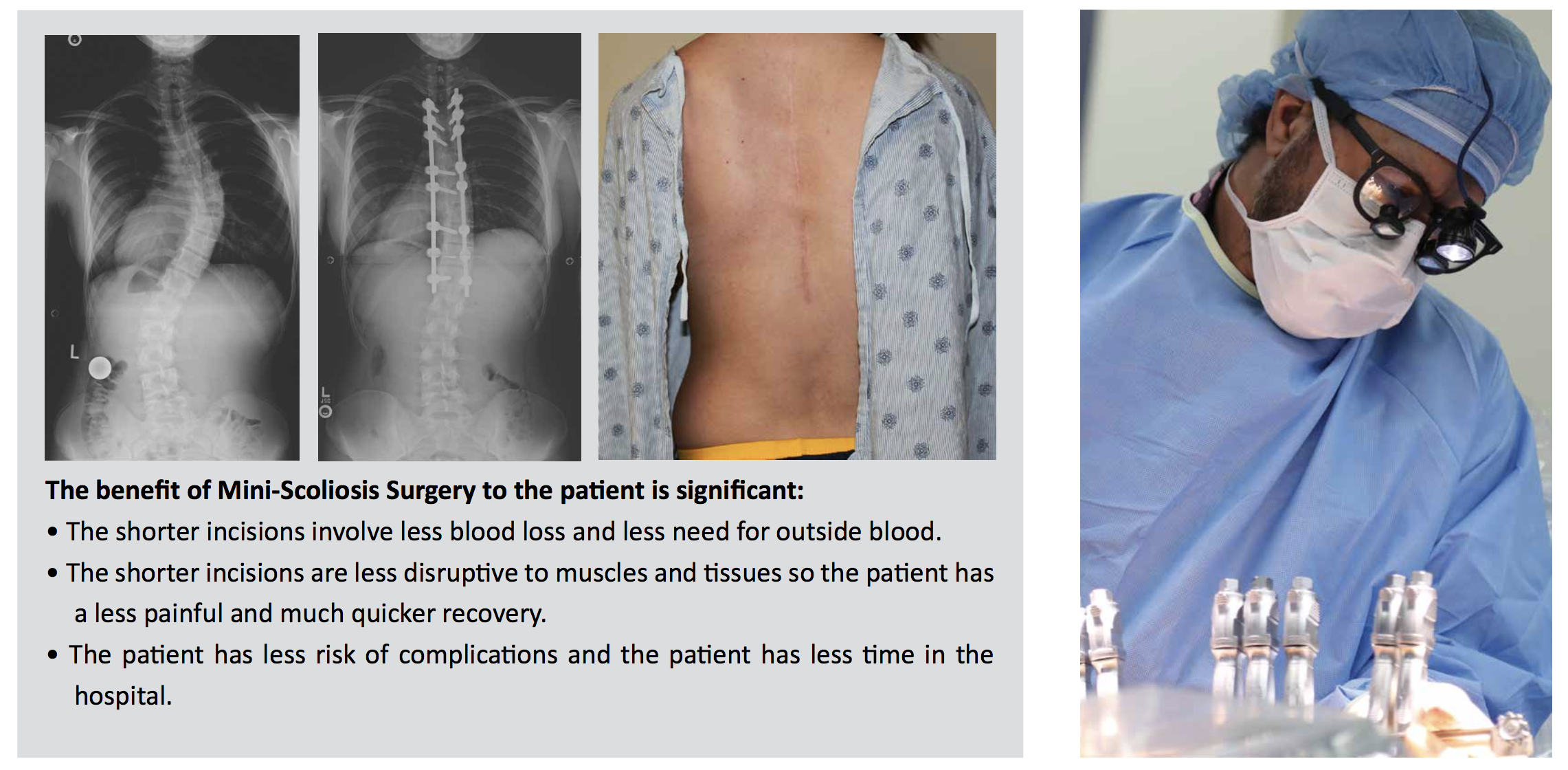
A: Over the past 100 years, significant advancements have been made in spinal fusion to treat scoliosis. These advancements have led to improved fusion rates, lower complication frequency, greater three-dimensional correction of the deformity and more rapid postoperative recovery, and extremely reliable surgical techniques. However, spine fusions may mean (in fusions below Lumbar level 2) fewer motion segments (less spine motion), which may lead to lower function in high-level physical activity (e.g. competitive athletics) and greater chance for spine arthritis. As a result, preservation of spinal motion, particularly in the low back, is an important goal.
The desire to maintain spine motion has fueled the development of various growth modulation procedures. The goals of these procedures are to correct the spinal deformity and maintain motion (without fusion). One of these promising techniques that has gained traction in the last 10 years is vertebral body tethering (VBT).
A: Studies on VBT in animal models have demonstrated proof of concept that tethering of the immature spine can alter its growth. The first description of VBT use in humans was a case report in 2010. Early, short-term, single institution studies have been encouraging with few reported serious complications. In addition, the FDA has just reported on (as of fall 2019) an additional dataset of 57 patients that showed correction of scoliosis and avoidance of spinal fusion in all but one. However, revisions for overcorrection and for tether breakage did lead to a higher reoperation rate than for gold standard 4th generation fusions.
A: Patients who can benefit from VBT include:
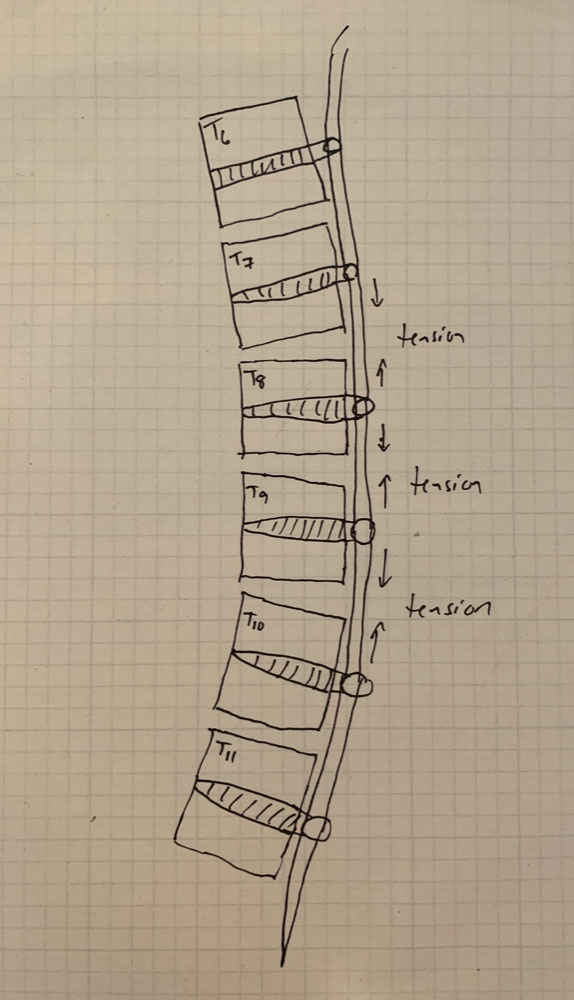 A: VBT is a thoracoscopic, minimally-invasive technique in which screws are placed into the vertebral bodies on the convex side of the coronal deformity. The screws are placed into the middle of the vertebral body with bicortical purchase under fluoroscopic guidance. A high-strength, braided polypropylene tether is then placed into the screw heads and then sequentially secured to each screw after segmental compression. The technique achieves modest correction of the spinal deformity immediately postoperative.
A: VBT is a thoracoscopic, minimally-invasive technique in which screws are placed into the vertebral bodies on the convex side of the coronal deformity. The screws are placed into the middle of the vertebral body with bicortical purchase under fluoroscopic guidance. A high-strength, braided polypropylene tether is then placed into the screw heads and then sequentially secured to each screw after segmental compression. The technique achieves modest correction of the spinal deformity immediately postoperative.
A: There are several. Placement of anterior body screws above T4 and below L4 is difficult. There are also questions about the number of vertebra that should be included into the VBT construct, how much tension to place across each vertebral motion segment within the VBT construct, optimal screw trajectory and screw size, placement of VBT across the diaphragm for thoracolumbar curves, and implant prominence.
A: Innovations, especially in the area of surgical spine deformity treatment and advances, can occur quickly. Physicians, and especially surgeons, espouse primum non nocere or “first, to do no harm” is a basic tenet of medicine. This is why for surgical procedures, such as VBT, safety is the pre-eminent concern, even more so than its efficacy or how well it works. If a surgical procedure is safe (infrequent, minor complications, with no significant long-term problems) but only demonstrates mild to moderate efficacy then it may be viewed as a reasonable treatment. However, if the procedure doesn’t have demonstrated, reasonable safety, it is unlikely any level of efficacy will be able to make this a reasonable treatment. This is especially the case for diseases which are not life-threatening, such as scoliosis.
Initial data, both from the FDA HDE cohort, the orthopedic literature, and abstract level data sets, indicate that vertebral body tethering provides benefit related to prevention of spinal curve progression and avoidance of spinal fusion.
A: As patients and caregivers consider if VBT is an appropriate treatment, it is important that the potential complications or adverse outcomes are detailed and well-understood as to their likelihood, severity and long-term implications.
The list of complications which may occur with VBT are:
The goal, for vertebral body tethering, is that reoperations for “failure”…that is eventual spinal fusion, should be less than 10% and with time, comparable to 4th generation techniques for spinal fusion (less than 3%).
A: The simplistic answer is we don’t know. We do have insight about what happens to the actual tether though.
If we look at other implant systems used in the spine and other bones of the body over the last 50-plus years we can roughly sketch out some possible scenarios for the system currently used for VBT. The fixation in the vertebra are screws that, as a group, have a long history of safety and efficacy. Based on the collective experience, it appears the screws have the same efficacy and safety profile and few issues with prominence, migration or pullout.
The other aspect of VBT is the tether, which is made of braided polypropylene. This is the workhorse of the system, which compresses across the convex discs and growth plates to modulate spine growth. Since there is no fusion across the vertebral bodies, there will be constant motion on the tether. Like any non-regenerating material that is constantly moving, the tether is subject to fatigue, which can lead to failure or breakage of the tether. It makes sense that the tether will eventually break considering it is implanted in adolescents and will likely be stressed for 60 years or more.
There have been reports of segmental failure of the tether (between two screws), so it is reasonable to assume that in the long-term the tether will likely break in multiple locations. This may not result in failure. For the sake of the aim of VBT to modulate growth in the immature spine, we only need it to last until the completion of spinal growth with hopefully permanent correction of the scoliosis. We don’t want the tether to break prior to this time, which would permit the spine deformity to worsen.
For your child to be considered for vertebral body tethering at ScoliosisTexas, they will need to meet certain criteria, including:
If you would like to find out if vertebral tethering may be an option for your child, contact us to set up an appointment to see one of our doctors, or set up an Econsult.
Vertebral Body Tethering requires a spine surgeon who specializes exclusively in scoliosis as tethering is a very specialized procedure done through very small incisions to reduce disruption to muscles and ligaments in the back. This enables the scoliosis patient to recover faster with a less painful return to activity.
 Zimmer Biomet makes technology related to the tethering procedure.
Zimmer Biomet makes technology related to the tethering procedure.
As with any new procedure, there is great hope that Vertebral Body Tethering can arrest a spinal curve in the young scoliosis patient and possibly eliminate the need for a more complex and invasive spine surgery. Tethering applies mostly to adolescent spinal curves that are still in the growth spurt stage.
Vertebral Body Tethering is in fact not a new concept and has much in common with scoliosis bracing which has been used as a standard treatment for spinal curves for decades.
Both bracing and Vertebral Body Tethering involve the concept of bone growth modulation which is based off the Hueter-Volkmann principle, which states that bone under more pressure will grow slower and denser than bone not under stress. So with bracing or tethering the bone on the inside part of the curve will grow slower and denser than the bone on the outer part of the curve which in turn creates a vertebrae more wedge shaped.
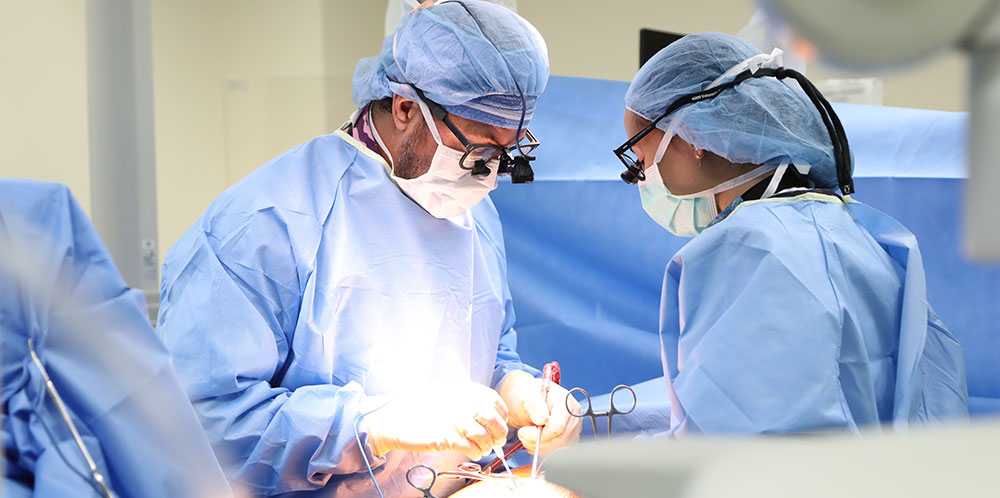
The difference between bracing and tethering is that the goal with bracing is to PREVENT THE SPINAL CURVE FROM WORSENING. It is important to understand that bracing does not correct the existing curve.
Conversely, with Vertebral Body Tethering loading is applied directly to the spine with a surgical procedure, which creates bone growth modulation and attempts to provide some correction of the spinal curve — if the patient has young with more bone growth in the future.
In this sense, Vertebral Body Tethering is an early intervention option for the adolescent scoliosis patient whose bones have not fully matured, rather than someone who is now an adult.
The benefits of Vertebral Body Tethering include:
The patient must young enough to still have bone growth remaining. This could be a child 8 years old. Girls still have bone growth up to age 14 while boys can still have bone growth up to age 16.
A candidate would have idiopathic scoliosis with curve less than 65 degrees who are generally flexible and whose bone structure is large enough to accept the installation of screws and anchors into the vertebrae. This is determined with an X-ray. The young patient should also have failed traditional bracing, or cannot tolerate wearing a brace.
 Who does NOT quality for Vertebral Body Tethering
Who does NOT quality for Vertebral Body TetheringThe Vertebral Body Tethering procedure should not be used for the following patients:
The Tethering System, developed by Zimmer Biomet Spine, was approved by the FDA in August 2019. The system is made up of anchors, bone screws, cord and set screws. The anchors, bone screws and set screws are made out of titanium alloys that are commonly used as spine implants. The cord is made of a strong flexible polymer.
During surgery, the scoliosis surgeon places the anchor and bone screw into the patient's spine on the side of the spinal curve. The polymer cord is then secured to the bone screws using set screws. The surgeon then applies tension to the cord to partially reduce the curve in the person's spine. After surgery, the cord continues to straighten the spine as the patient continues to grow.
It’s important to remember that tethering carries similar risks to any invasive surgery, including the use of general anesthesia.
With Vertebral Body Tethering the surgeon accesses the spine through the chest. During surgery, the patient's lung on that side of the chest is temporarily deflated. After the tether has been installed, the lung is then re-inflated. One of the recovery aspects is that there will be soreness and pain after surgery, making it uncomfortable to take deep breaths or cough. Ultimately the chest tube is removed and the soreness disappears.
Recovery can be fairly quick with the young patient being released to return to activity and to athletics about a month to six weeks after surgery.
Research conducted in clinical trials which resulted in FDA approval of the surgical procedure indicates that Vertebral Body Tethering can be successful in arresting a curve and in some cases eliminating the need for spinal fusion or instrumentation in the future, which makes it a viable option for the child or adolescent scoliosis patient.
FDA approval was based on clinical data from 57 patients who underwent Vertebral Body Tethering. Data after two years revealed that 43 patients had sufficient improvement in the curvature of the spine and did not require spinal fusion.
Dr. Matthew Geck, renowned scoliosis surgeon, based in Austin, Texas is the second approved site behind Mayo Clinic to participate in the clinical study assessing the latest BRAIVE vertebral body tether designed by Medtronic.
The Braive study will evaluate the safety and effectiveness in treating adolescent idiopathic scoliosis that is worsening. The Braive system system involves a rope-like braided tether inserted through fixation instrumentation attached to the vertebrae in the spine. Unlike metal instruments that are routinely used in adult scoliosis, the braided tether enables the spine in the adolescent to continue to grow while preventing the curve from worsening.
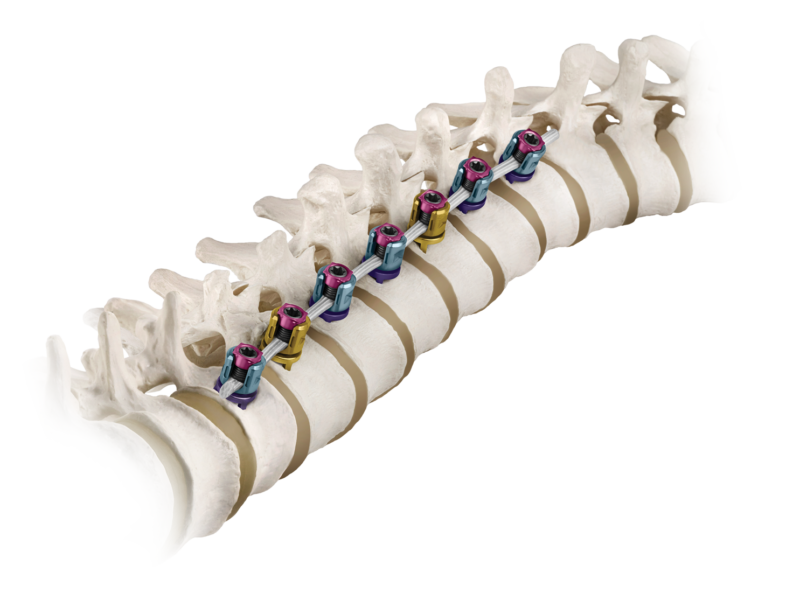
The hope with vertebral tethering technology in a teenager is that the spinal curve can be arrested while the spine is going through it’s most significant growth period. This may make further scoliosis surgery unnecessary, or less extreme and less risky if it needs to be performed when the patient becomes an adult.
The Medtronic pediatric spine device uses a braid secured to the spine to slow the growth of the curved side of the spine. The clinical study will include patients in the U.S., Canada and the United Kingdom. The first surgery to implant the new BRAIVE technology was done in the United Kingdom at the Newcastle Upon Tyne Hospital.
According to Medtronic, around 4% of children across the globe have scoliosis, which occurs when the vertebrae twist or rotate that may cause the spine to curve into a “C” or “S” shape rather than a straight line. Using a braided tether secured to the spine with screws, the Braive system helps to slow growth on the curved side of the spine while enabling to continue the growth on the other side. The clinical trial will assess whether the system is safe and effective in correcting the spine’s curve in patients with juvenile or adolescent idiopathic scoliosis.
“The goal of the new BRAIVE tether technology is to provide a highly reliable tether that is strong enough to last through adolescence, lessening the risk of the braid fraying or snapping,” according to scoliosis surgeon, Dr. Matthew Geck.
According to the National Scoliosis Foundation, an estimated 30,000 children each year receive a brace in an attempt to arrest the spinal curve. Another 38,000 patients are treated with spinal fusion that locks vertebrae in place. Spinal fusion, however, causes vertebrae to fuse together into a single bone, which stops growth in that area of the spine.
The Braive growth modulation system avoids fusion by using a rope-like braid secured to the spine with screws. This in turn slows the growth on the curved side of the spine, while allowing growth to continue on the other side. The BRAIVE clinical study will evaluate whether the system is safe and effective in correcting the spine's curve in pediatric patients and teenagers with scoliosis.
Within a clinical study, strict criteria is followed related to which pediatric or adolescent scoliosis patients qualify for the last vertebral tether technology.
A subject must meet all of the following INCLUSION CRITERIA to participate in this study:
Additionally the study has specific EXCLUSION CRITERIA, including but not limited to the following:
Patients can contact Dr. Geck’s office related to accessing the clinical study by calling Texas Spine and Scoliosis at 512-324-3580. More information about Dr. Geck is at TexasSpineandScoliosis.com and ScoliosisTexas.com.
Click here to download |
Click here to download |
Texas Spine and Scoliosis is a regional referral center for the treatment of back and neck pain and scoliosis

Learn more about the providers at Texas Spine and Scoliosis, the only spine specialized neurosurgery, orthopedic surgery, and non-surgical rehabilitation group in the central Texas area.
 Texas Spine & Scoliosis approved for the new BRAIVE scoliosis tethering study
Texas Spine & Scoliosis approved for the new BRAIVE scoliosis tethering study

Read about various patient success stories that have been performed by the physicians at Ascension Texas Spine & Scoliosis